<<
Back to Kanarev's Physchemistry Book Index
9.25.
Structure of the Nucleus of the Titanium Atom
Titanium is situated in the fourth group of the
periodic table; that’s why the nucleus of the carbon atom should be repeated in
the structure of its nucleus (Fig. 26). In the nature, there are 8.20% of the
nuclei of the titanium atom containing 22 protons and 24 neutrons. There are 22
protons and 25 neutrons in 7.40% of the nuclei, 73.80% of the nuclei have 22
protons and 26 neutrons. The number of the nuclei having 27 neutrons is 5.40%
and 28 neutrons – 5.20%. In Fig. 42, there is a diagram of the nucleus of
titanium atom, in which there are 22 protons and 24 neutrons [120].
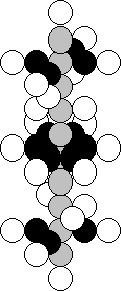
Fig. 42. Diagram of
the nucleus of the titanium atom
9.26. Structure of
the Nucleus of the Vanadium Atom
Vanadium is the twenty third element in the periodic table.
It is situated in the fifth group of this table, that’s why the nucleus of the
nitrogen atom should be in the structure of its nucleus (Fig. 27). The majority
of the nuclei of the atoms of this element contain 23 protons and 28 neutrons
(Fig. 43) [120].
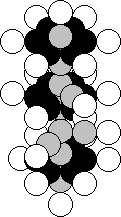
Fig. 43. Diagram of
the nucleus of the vanadium atom
9.27.
Structure of the Nucleus of the Chromium Atom
Chromium is situated in the sixth group of the periodic
table. The majority of the nuclei of the atoms of this element contain 24 protons
and 28 neutrons (Fig. 44) [120].
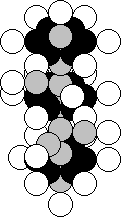
Fig. 44. Diagram of
the nucleus of the chromium atom
9.28.
Structure of the Nucleus of the Manganese Atom
Manganese is the twenty fifth element in the periodic table.
It is situated in the seventh group of this table. One hundred per cent of the
atoms of this element contain the nuclei with 25 proton and 30 neutrons (Fig.
45) [120].
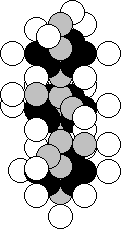
Fig. 45. Diagram of
the nucleus of the manganese atom
9.29.
Structure of the Nucleus of the Iron Atom
Iron (Fig. 46) is the twenty sixth element in the
periodic table. The majority of the atoms of this element contain the nuclei
with 26 protons and 30 neutrons (Fig. 46) [120].

Fig. 46. Diagram of
the nucleus of the iron atom
9.30.
Structure of the Nucleus of the Cobalt Atom
 One hundred per cent of the atoms
of this element contain the nuclei with 27 proton and 32 neutrons (Fig. 47)
[120].
One hundred per cent of the atoms
of this element contain the nuclei with 27 proton and 32 neutrons (Fig. 47)
[120].
Fig. 47. Diagram of the
nucleus of the cobalt atom

Fig. 48. Diagram of
the nucleus of the nickel atom
9.31.
Structure of the Nucleus of the Nickel Atom
Nickel is situated in the eighth group of the periodic
table. The majority of the atoms of this chemical element contain 28 protons
and 30 neutrons (Fig. 48).
9.32. Structure of the Nucleus of
the Copper Atom
The copper atom is situated in
the first group of the fourth period of the periodic law of chemical elements.
It means that a marked nucleus of the lithium atom should be present in the
structure of the nucleus of this element (Fig. 23). A stable nucleus of this
atom, and there are 69.17% of such ones, contains 29 protons and 34 neutrons
(Fig. 49). As it is clear, the nucleus of the lithium atom is arranged at the
top of the nucleus of the copper atom.
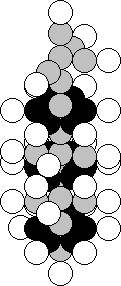
Fig. 49. Diagram of
the nucleus of the copper atom
9.32. Brief
Conclusions
1. One can suppose that a principle of the construction of
the atomic nuclei of chemical elements has been found. In the atomic nucleus,
the neutrons and the protons are connected by magnetic forces of their magnetic
poles. The proton has the simplest magnetic field similar to magnetic field of
the bar cylindrical magnet. The neutron has a compound magnetic field, which
forms on its surface six symmetrically arranged magnetic poles: three south
poles and three north ones.
2. The nucleus of any chemical element is formed in such a
way that there should be a neutron between the protons, which connects the
protons and plays the role of a screen between the like electric fields of the
protons.
3. The above-mentioned method of the construction of the
nuclei of the atoms of chemical elements gives the opportunity to build a
nucleus of any atom. It is clear that the flat nucleus of this atom serves as a
base for the nuclei of all atoms, which are more complicated than the carbon
atom. Further progress will show that the flat components similar to the flat
nucleus of the carbon atom will take place in succession. Complexity of the
nuclear structure will be determined by a number of the nuclei of the carbon
atom in it [121].
4. We understand that during further investigations of the
nuclear structures the atomic nuclei of all eight groups of the first period
and of the second period will be the closest ones to reality. The structures of
more complicated nuclei will be specified.
The
Foundations of Physchemistry of Microworld
Copyright Ó2003 Kanarev Ph.
M.
Internet Version - http://book.physchemistry.innoplaza.net
<< Back to Physchemistry Book Index