Published 25.09.2003. Updated 05.10.2003.
Ph. M. Kanarev,
A.I. Tlishev, D.A. Bebko
E-mail: kanphil@mail.kuban.ru
GENERATORS OF GLOBAL
(CLEAN) ENERGY
INTRODUCTION
The global energy
problems have been discussed for a long time; they are well known. Depletion of
natural energy carriers (oil, gas and coal) is the first problem. Environmental
safety of energy carriers is the second problem. It is generally recognized
that the solution of the issues connected with the aggregate of these two
problems is of a global character. The scientific research results, which
should belong to global (clean) energy, originate from it. First and foremost,
they are such scientific investigations, which result in the possibility to use
an inexhaustible and environmental friendly energy carrier. It has been known
for a long time. It is hydrogen produced from water.
Why hydrogen and
why from water? Because when hydrogen is burnt, water is formed again; thus,
the energy carrier is inexhaustible. As far as inexhaustibility and
environmental safety are concerned, hydrogen has no competitors. But the
implementation of these qualities is restricted by large energy expenses for
hydrogen production from water. For hydrogen production, modern electrolyzers
use by 10 or 20% more energy when it is produced while hydrogen is burnt.
It is easy to
imagine what financial and intellectual resources of the world are included in
the search of the ways how to reduce energy expenses for hydrogen production
from water. In Russia, many scientific institutions of branch science and
educational institutions are busy with this problem. There exists a research
hydrogen institute. The associations of scientists on hydrogen energetics have
been established in USA and Europe.
Energy problem is
global not due to depletion of oil and gas, but due to their environmental
hazard. But the confirmations exist that the world owners of energy resources
do not bother about the environmental hazard problem of modern energy carriers.
In history of
science, the facts of annihilation of the scientists who have success in energy expense reduction for
hydrogen production from water have already been registered. The orderers of
these actions think that they will lose their profits with the rise of hydrogen
energetics. They do not understand that this rise cannot be abrupt. It is
impossible to substitute hydrogen energetics infrastructure for energy
infrastructure of the existing energy carriers during one year or even during
ten years. Besides, hydrogen energetics infrastructure will be created not on a
blank space. It will be integrated into the existing infrastructure of
energetics gradually; and its owners will automatically become the owners of
hydrogen energetics. The first step has already been made in this direction. In
USA, a decision has been adopted to equip all filling stations with the pumps
to fill the cars with hydrogen.
It is known that
the theoretical investigation results publication in press is a priority.
Usually, such priority is a personal one. Usually, a patent is a priority of
the experimental investigation results. As a rule, this patent belongs to a
group of authors. A published patent is a jinnee released from a bottle. No
finesse of the authors to encumber a reproduction of experimental data given in
a patent without the participation of the authors can stop the process of their
implementation. Thus, the authors or a group of the authors who have filed an
application for a patent are deprived automatically of the opportunity to
influence the process of practical realization of their ideas.
It is known that if
it becomes possible to reduce energy expenses for hydrogen production of water
5fold, it will be the cheapest energy carrier. Russia has already got
technology, which reduces these expenses 10fold and more. But another direction
is more perspective. What is the use to break down water into hydrogen and
oxygen and to use hydrogen as fuel for heating, for example, water in heating
systems? Is it possible to make water generate heat? As it happens, it is
possible.
In Russia, three
firms (Yusmar, Termovikhr and Noteka) sell cavitation heating equipment with
energy performance index up to 150%. Official science looks awry at this
activity, because such results conflict with one of the main laws of physics:
law of conservation of energy. But market profit is stronger than this law.
Engineering
practice has already proved that additional energy in the form of heat is
generated in the ventilation systems and in the water cavitation systems. Deep
scientific analysis of this problem shows that physical vacuum is the most
probable source of additional energy in the systems of ventilation and water cavitation.
Valence electrons of destroyed molecules of water take energy from physical
vacuum and release it during repeated fusion of these molecules.
Why is additional
energy generated in the air systems of ventilation and in water cavitation
systems? Because they are mechanical systems; mechanical destruction of
chemical links requires half energy as compared with thermal destruction of
these links. This is the main reason why one fails to increase energy
performance index of cavitation processes over 200%.
An increase of
efficacy of any process by 30 or 50% is a good result; if it is obtained, it is
possible to get even better result. What if a water molecule is destroyed not
mechanically, but electrodynamically? In this case, it becomes possible to find
resonance frequencies of impact on the molecules and to reduce considerably
electrical energy expenses for their destruction. Subsequent fusion of
destroyed molecules will release prescribed quantity of energy unavoidably. It
is a simple idea, and it has already been implemented (Tabl. 4-6).
In order to get results given below, it is
necessary to get new microworld physchemistry knowledge, which has already been
published. Every month more than 1000 foreign scientists get acquainted with
this knowledge visiting the site http://Kanarev.innoplaza.net
The Russian
speaking readers get this information on the sites http://www.ikar.udm.ru/sb28-2.htm and http://www.n-t.org/tp/ts/eb.htm
LOW CURRENT PROCESS OF
WATER ELECTROLYSIS
Low voltage process of water electrolysis is known from Faradays times.
It is widely used in modern industry. Voltage of 1.6-2.3 volts is operation
voltage between the anode and the cathode of the electrolyzer; current strength
is tens and hundreds of amperes. In accordance with Faradays law, energy
consumption for production of one cubic meter of hydrogen is nearly 4 kWh in this case.
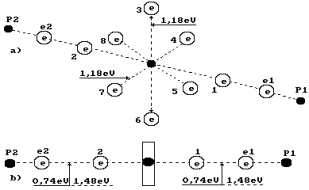
The analysis of the water molecule structure (Fig. 1)
worked out by us shows the possibility of water electrolysis at minimal current
and even without it. The protons of the hydrogen atoms in water molecules can
be combined with each other and can form clusters. As a result, an
orthohydrogen molecule is formed (Fig. 2). A question arises: is it possible to
separate this molecule from such cluster? The results of answers on this
question are given in Tables 1, 2 and 3.
|
Fig. 1. Water molecule diagram: 1,2,3,4,5,6,7,8 are numbers of
the electrons of the oxygen atom; P1, P2 are the hydrogen atom nuclei (the
protons); e1 and e2 are the electron numbers of the hydrogen atoms |
Fig. 2. Formation diagram of orthohydrogen: a) and b) water molecule
diagrams; c) orthohydrogen |
It is known that a gram-atom is equal to atomic mass
of substance; a grammolecule is equal to molecular mass of substance. For
example, the grammolecule of hydrogen in the water molecule is equal to two
grams; the gram-atom of the oxygen atom is 16 grams. The grammolecule of water
is equal to 18 grams. Hydrogen mass in a water molecule is 2x100/18=11.11%;
oxygen mass is 16x100/18=88.89%; this ratio of hydrogen and oxygen is in one
liter of water. It means that 111.11 grams of hydrogen and 888.89 grams of
oxygen are in 1000 grams of water.
One liter of hydrogen weighs 0.09 g; one liter of
oxygen weighs 1.47 g. It means that it is possible to produce 111.11/0.09=1234.44
liters of hydrogen and 888.89/1.47=604.69 liters of oxygen from one liter of
water. It appears from this that one gram of water contains 1.23 liters of
hydrogen. Energy consumption for production of 1000 liters of hydrogen is 4 kWh
and for one liter 4 Wh. As it is possible to produce 1.234 liters of hydrogen
from one gram of water, 1.234x4=4.94 Wh is spent for hydrogen production from
one gram of water now.
Instruments
and equipment used during the experiment
Special experimental low current electrolyzer (Fig.
3); voltmeter of the highest accuracy class (accuracy class of 0.2 GOST
9711-78); ammeter of the highest accuracy class (accuracy class of 0.2 GOST
9711-78) electronic scale with scale division value of 0.1 and 0.01 g; stop
watch with scale division value of 0.1 s.

Fig. 3. Low current electrolyzer in the
closed form (in the process of patenting)
Table 1
|
Indices |
Sum |
|
1
- duration of the experiment t, h |
6.000 |
|
2
readings of voltmeter V, volts |
3.750 |
|
3
ammeter readings I, amperes |
0.020 |
|
4
power P, watts hour (P=VxIxτ/60) |
0.450 |
|
5
continue of experiment without input energy in 6 series, min |
0.000 |
|
6
mass difference, grams |
0.52 |
|
7
mass of evaporated water, grams |
0.01x6=0.06 |
|
8
mass of water converted in hydrogen |
0.46 |
|
9
specific power P=P/ |
0.98 |
|
10
existing specific power P,
Watt/gram of water |
4.94 |
|
11 the reducing
power on the production of hydrogen, times K=P/P |
5.04 |
|
12
quantity of released hydrogen, ΔМ
=0.46x1.23x0.09=0.051, grams |
0.051 |
|
13
energy content of hydrogen being obtained (Е=0.051х142/3,6)=2.008
Wth |
2.008 |
|
14-
energy efficacy of low ampere process of water electrolysis (Eх100/P), % |
446.2 |
Table 2
|
Indices |
Sum |
|
1
- duration of the experiment with input energy in 6 series t, min |
6x30=180.0 |
|
2
readings of voltmeter V, volts |
3.750 |
|
3
ammeter readings I, amperes |
0.022 |
|
4
power P, watts hour (P=VxIxτ/60) |
0.247 |
|
5
continue of experiment without input energy in 6 series, min |
6x30=180.0 |
|
6
mass difference, grams |
0.45 |
|
7
mass of evaporated water, grams |
0.1x6=0.06 |
|
8
mass of water converted in hydrogen |
0.39 |
|
9
specific power P=P/ |
0.63 |
|
10
existing specific power P,
Watt/gram of water |
4.94 |
|
11 the reducing
power on the production of hydrogen, times K=P/P |
8.40 |
|
12
quantity of released hydrogen, ΔМ
=0.39x1.23x0.09=0.043, grams |
0.043 |
|
13
energy content of hydrogen being obtained (Е=0.043х142/3,6)=1.70
Wth |
1.70 |
|
14-
energy efficacy of low ampere process of water electrolysis (Eх100/P), % |
689.0 |
Table 3
|
Indices |
Sum |
|
1
- duration of the experiment with input energy in 6 series t, min |
6x5=30 |
|
2
readings of voltmeter V, volts |
13.60 |
|
3
ammeter readings I, amperes |
0.020 |
|
4
power P, watts hour (P=VxIxτ/60) |
0.136 |
|
5
continue of experiment without input energy in 6 series, min |
6x55=330 |
|
6
mass difference, grams |
0.44 |
|
7
mass of evaporated water, grams |
0.01x6=0.06 |
|
8
mass of water converted in hydrogen |
0.38 |
|
9
specific power P=P/ |
0.358 |
|
10
existing specific power P,
Watt/gram of water |
4.94 |
|
11 the reducing
power on the production of hydrogen, times K=P/P |
13.80 |
|
12
quantity of released hydrogen, ΔМ
=0.38x1.23x0.09=0.042, grams |
0.042 |
|
13
energy content of hydrogen being obtained (Е=0.042х142/3,6)=1.66
Wth |
1.66 |
|
14-
energy efficacy of low ampere process of water electrolysis (Eх100/P), % |
1220.0 |
Note: In Tables 1, 3, the results of the experiment
are given when frequency of nearly 500 Hz has been generated in the power
supply, in table 2 without frequency.
First of all, we should note that the anode and the
cathode are made of one and the same material: steel. It excludes the
possibility of formation of a galvanic cell. If we analyze Tables 1, 2 and 3,
well see the electrolysis process takes place at very low current of 0.02 A;
thats why it has been called low current one. Further, this process consisted
of two cycles in some experiments; in one cycle, the electrolyzer is connected
to the power line; in another cycle, it is disconnected (Tables 2, 3).
Gas generation process is manifested by release of the
bubbles being formed. The bubbles go on being released after the electrolyzer
is disconnected from the supply line (Tables 2 and 3). When the electrolyzer is
de-energized, gas release intensity is reduced, but it is not stopped during
many hours. It is proved by the fact that electrolysis takes place at the
expense of potential difference on the electrodes.
After electrolyzer de-energizing, gas release during a
long period of time proves the fact that the molecules of oxygen and hydrogen
are formed without the electrons emitted by the cathode, i.e. at the expense of
the electrons of the water molecule itself.
Simplicity and 100% reproducibility of the experiments
being described afford ground for the fact that mankind has got a chance to
avoid energy famine and environmental crisis.
WATER ELECTRIC
GENERATOR OF HEAT
We have already shown that
energy of physical vacuum taken by valence electrons of the molecules after
their mechanical destruction and emitted by these electrons within the repeated
fusion of the molecules is the most probable source of additional energy
generated by the ventilation systems and the cavitation ones. It is explained
by the fact that half as much energy is spent for mechanical destruction of the
molecules than for thermal destruction of these molecules. Valence electrons of
the molecules being destroyed mechanically absorb energy from physical vacuum
in order to restore their energy indices and emit it during the repeated fusion
of these molecules.
As half as much energy is spent for mechanical destruction of the
molecules than for thermal one, energy effectiveness index of such processes
cannot exceed two. But if this hypothesis is correct, there is a possibility to
increase energy effectiveness index of this process considerably when the
molecules are destroyed electrodynamically. In this case, there is a
possibility to find resonance modes of electrodynamic destruction of the molecules
and to reduce energy consumption for this process considerably. Further fusion
of the molecules being destroyed electrodynamically will release required
quantity of energy, which will exceed considerably the energy being spent.
EXPERIMENTAL PART
The
main task of the experiment was to check the hypothesis: "Electrodynamic
influence on the water molecules gives the possibility to reduce energy
expenses on destruction of their chemical bonds significantly; further fusion
of these molecules increases considerably the output of additional energy in
the form of heat".
In order to solve
this task, special experiments were carried out connected with electrodynamic
destruction of chemical bonds of water molecules with electric pulses of
various frequencies. The diagram of the installation used for experimental
investigations is shown in Fig. 4; the photo of the experimental generator of
heat is shown in Fig. 5.
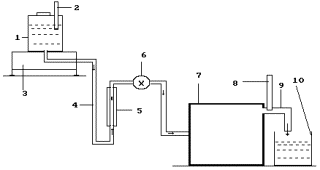
Fig. 4. Diagram of the experimental installation: 1 -
reservoir for solution; 2- thermometer; 3- electronic scales; 4 - solution feed
duct; 5- rotameter; 6- solution feed regulator; 7-a special thin plasma
generator is in the process of patenting; 8 - thermometer; 9- heated solution
discharge.
|
|
|
Fig. 5. Photos of heat generator
The results of the
experiments are given in Figs 6-9 and
in Tables 4 -5.
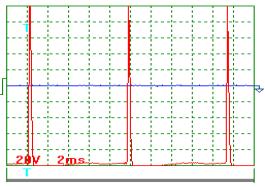
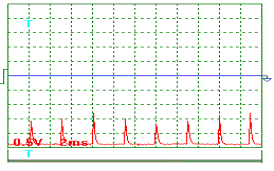
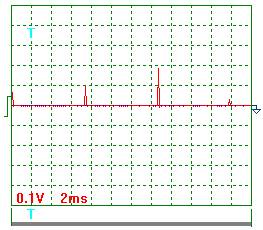
The oscillogram of voltage pulses is given in Fig. 6; the oscillogram of current pulses
influencing the generator of heat in one of the experiments carried out with
pulse frequency of nearly 100 Hz is given in Fig. 7. As it is clear from the
oscillograms, the pulses of both voltage and current have an exponential form
being close to the triangular one with a sharp edge and a shallow declination.
The design duty factor for these pulses is Z » 0.039. Mean amplitude of voltage pulses is equal to power supply
voltage of the pulse generator: 250 V. Thus, a mean component of voltage pulses
being brought to the generator of heat is equal to ![]() = 0.039 х 250 =
9.75 V. In this experiment, voltmeter readings were 10.0 V.
= 0.039 х 250 =
9.75 V. In this experiment, voltmeter readings were 10.0 V.
|
Fig. 6.
Oscillogram of power supply voltage pulses at |
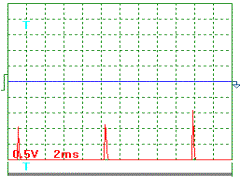
Fig. 7.
Oscillogram of power supply current pulses at |
A
current pulse oscillogram in this experiment is shown in Fig. 7. Current was
measured as voltage drop at a measuring resistor with resistance of 0.1 ohm included into the supply circuit of generator
of heat. As it is clear, mean amplitude of current pulses is 1.3 / 0.1 = 13
А, and mean component value is equal to: ![]() = 0.039 х 13 =
0.51 А. During measurements, the ammeter showed current of 0.50 A.
= 0.039 х 13 =
0.51 А. During measurements, the ammeter showed current of 0.50 A.
On the basis of the oscillographic measurement data,
mean value of electric power has proved to be Р = 9.8х0.51 = 5.0 W.
The experiment lasted for 300 seconds. Thus, electric energy of ![]() =5.0 х 300 = 1500 J = 1.5 kJ entered the generator of
heat. During this period, it heated 0.55 kg of solution by 12 degrees. Energy
value of this heat was equal to
=5.0 х 300 = 1500 J = 1.5 kJ entered the generator of
heat. During this period, it heated 0.55 kg of solution by 12 degrees. Energy
value of this heat was equal to ![]() =4.19х0.55х12=27.65 kJ. Efficiency index of
energy process was К = Е2 / Е1 = 27.65 / 1.5 = 18.43, or 1843%.
It corresponds (with accuracy of up to 5% being characteristic of
oscillographic check) to energy efficiency index being determined with the help
of the voltmeter and the ammeter (s. Table 4).
=4.19х0.55х12=27.65 kJ. Efficiency index of
energy process was К = Е2 / Е1 = 27.65 / 1.5 = 18.43, or 1843%.
It corresponds (with accuracy of up to 5% being characteristic of
oscillographic check) to energy efficiency index being determined with the help
of the voltmeter and the ammeter (s. Table 4).
Table 4
Experimental
indices of the water electric generator of heat with electric pulse frequency of nearly 100 Hz
|
Indices |
Mean |
|
1.
Mass of the solution, which has passed through the generator |
0.55 |
|
2.
Temperature of solution at the input of the generator |
26.00 |
|
3.
Temperature of the solution at the output of the generator |
38.00 |
|
4.
Temperature difference of the solution
|
12.00 |
|
5.
Durability of the experiment |
300.00 |
|
6.
Reading of voltmeter |
10.50 |
|
6.
Reading of oscillograph |
9.75 |
|
7.
Reading of ammeter |
0.50 |
|
7.
Reading of oscillograph |
0.51 |
|
8.
Electric power consumption, |
1.50 |
|
9
power spent for heating of the solution |
27.65 |
|
10 reactor efficiency index |
18.43 |
In Fig.
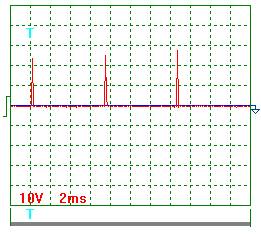
8, the oscillogram of voltage pulses is given. In Fig. 9, the oscillogram of
current pulses being registered during another experiment with pulse frequency
of nearly 300 Hz is given. According to these oscillograms, the duty factor
calculation has given the result of Z = 0.11. With mean values of amplitudes of
pulses of voltage and current being equal to 250 V and 10.6 A, respectively,
the mean components of voltage and current arriving into the generator of heat
have been: ![]() = 0,11 х 250 =
27.5 V;
= 0,11 х 250 =
27.5 V; ![]() = 0.11 х 10.6 =
1.17 A. According to the readings of the voltmeter and the ammeter, mean values
of voltage and current were 25.0 V and 1.25 A in this experiment. In this
connection, mean value of electric power supplied to the generator of heat was
27.5 х 1.17 = 32.18 W according to the data of the oscillographic
measurements and 25 х 1.25 = 31.25 W according to the data of the pointer
indicators. Divergence in this methods of mean power determination did not
exceed 5% as well.
= 0.11 х 10.6 =
1.17 A. According to the readings of the voltmeter and the ammeter, mean values
of voltage and current were 25.0 V and 1.25 A in this experiment. In this
connection, mean value of electric power supplied to the generator of heat was
27.5 х 1.17 = 32.18 W according to the data of the oscillographic
measurements and 25 х 1.25 = 31.25 W according to the data of the pointer
indicators. Divergence in this methods of mean power determination did not
exceed 5% as well.
The energy efficiency calculation results of the generators of the heat
for both methods of measurement with pulse frequency of nearly 300 Hz are given
in Table 5. They are close in their values as well.
|
Fig. 8. Oscillogram of supply voltage pulses at |
Fig. 9. Oscillogram of supply current
pulses at |
Table 5
Experimental
indices of the water electric generator of heat with electric pulse frequency of nearly 300 Hz
|
Indices |
Mean |
|
1. Mass of the solution, which has passed through
the generator |
0.41 |
|
2. Temperature of solution at the input of the
generator |
26.00 |
|
3. Temperature of the solution at the output of the
generator |
76.00 |
|
4. Temperature difference of the solution |
50.00 |
|
5. Durability of the experiment |
300.00 |
|
6. Reading of voltmeter |
25.00 |
|
6. Reading of oscillograph |
27.5 |
|
7. Reading of ammeter |
1.25 |
|
7. Reading of oscillograph |
1.17 |
|
8. Electric power consumption, |
9.38 |
|
9. Power spent for heating of the solution |
85.90 |
|
10.
Generator efficiency index |
9.16 |
PROTOCOL OF CONTROL TEST
Table 6
Supply voltage and current were measured with the help
of a voltmeter, an ammeter and an oscillograph
(Fig. 10-13)
|
Indices |
1 |
2 |
3 |
Mean |
|
1 mass of the solution, which has passed through
the reactor m, kg. |
0.470 |
0.432 |
0.448 |
0.450 |
|
2 temperature of solution at the input of
the reactor t1, degrees |
22 |
22 |
22 |
22 |
|
3 temperature of the solution at the output
of the reactor t2, degrees |
66 |
66 |
65 |
65.67 |
|
4 temperature difference of the solution Dt= t2 - t1, degrees |
44 |
44 |
43 |
43.67 |
|
5 durability of the experiment Dt, s |
300 |
300 |
300 |
300 |
|
6 reading of voltmeter V, V |
4.50 |
4.50 |
4.50 |
4.50 |
|
6.
Reading of oscillograph |
4.47 |
4.47 |
4.47 |
4.47 |
|
7 reading of ammeter I, A |
2.1 |
2.1 |
2.1 |
2.1 |
|
7.
Reading of oscillograph |
2.2 |
2.2 |
2.2 |
2.2 |
|
8 electric power consumption according to indices
of voltmeter and ammeters, E2=IΧVΧDt, kJ |
2.84 |
2.84 |
2.84 |
2.84 |
|
9 power spent for heating of the solution,
E3=4.19ΧmΧDt, kJ |
79.64 |
80.01 |
80.72 |
80.46 |
|
10 reactor efficiency index K= E3/
E2 |
28.04 |
28.17 |
28.42 |
28.21 |
|
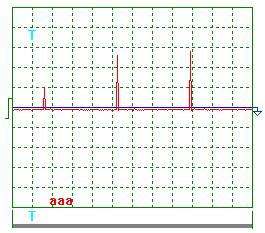
Fig. 10. Tension |
Fig. 11. Tension |
|
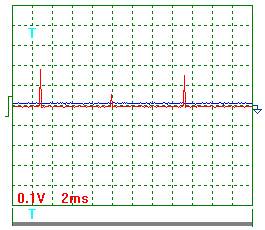
Fig. 12. Current |
Fig. 13. Current |
Process parameter calculation according to the
oscillograms (Fig. 10-13) to the check test protocol (Table 6) gave the
following results.
Pulse scale 10.
Mean voltage amplitude according to Fig.10 and Fig.
11:
Ua = (23+25+28+10+26+29) х 10/6 = 235 V.
Mean current amplitude according to Fig. 12 and Fig.
13:
Iа = (20+6+17+7+10+19+3) х 10/7 = 117 A.
Pulse repetition period T = 7.4 ms.
Pulse duration t = 0.28 ms.
Pulse frequency f = 1000/7.4 = 135.1 Hz.
Relative pulse duration S = 7.4/0.28 = 26.32.
Space factor Z = 0.5/26.32 = 0.019.
Mean value of pulse voltage импульсов Um =
0.019 х 235 =
4.47 V.
Mean value of current in pulses Im = 0.019 х 117 = 2.22 A.
Thus, it is possible to consider that an experimental
check of energy efficiency of the water electric generator of heat with the
help of two methods gives practically the same results and confirms the
above-mentioned hypothesis concerning the possibility of additional energy
production in the processes being considered. It can be noted
that as during measurements the pointer instruments of high class of accuracy
of 0.2 have been used (relative conventional gauging error does not exceed
0.2%) and oscillographic measurement accuracy is much lower (usually nearly
5%), the readings of the voltmeter and the ammeter should be considered as more
accurate ones.
Commercial efficiency of the
water electric generator of heat will depend on pulse generator economies. As
efficiency of powerful pulse generators can be near unit, energy efficiency
should not differ greatly from the data being obtained during laboratory
investigations for the industrial-scale plants with the use of the generators
of heat being considered.
The analysis of energy balance of the molecules with covalent bonds
shows the possibility of formation of additional thermal energy with the energy
efficiency index, which exceeds unit greatly, and the experiments confirm this hypothesis
earnestly.
Simplicity and hundred per cent reproducibility of the experiments being
described open a prospect for quick commercialization of the water electric
generator of heat.
CONCLUSION
Thus, the convincing theoretical and experimental proofs of existence of
a method, which reduces energy consumption for hydrogen production from water
10folds and more, have been got.
The method of conversion of electric energy into thermal energy with energy
efficiency index of more than 2000% has been found.
The way of a transfer to economical and environmental friendly power
engineering is opened. But it will not be an easy one. There will be a lot of
work concerning optimization of the parameters of the global energy generators.
REFERENCES
1. Kanarev Ph.M. The Foundation of Physchemistry of Microworld (the
second edition). (In
Russian) http://www.ikar.udm.ru/sb28-2.htm
2. Kanarev Ph.M. The Foundation of
Physchemistry of Microworld.
The second edition. (In English). http://book.physchemistry.innoplaza.net
3. Kanarev Ph.M., G.P. Perekoty, D.A. Bebko, A.A.
Chernyavsky. Water Electric Generator of Heat. http://kanarev.heatgenerator.innoplaza.net
4. Kanarev Ph.M. Energy Balance of Fusion
Process of Oxygen, Hydrogen and Water Molecules. New Energy Technologies. 2003,
Issue No. 3 (12), pages 58-62. (In English).
5. Kanarev Ph.M. Global Energy. New Energy Technologies.
2003, Issue No. 3 (12), 2003, pages 56-57. (In English).
6. Kanarev Ph.M. The Foundation of Physchemistry of Micro World. The second edition. (In English). http://book.physchemistry.innoplaza.net
7. Kanarev Ph.M. Energy Balance of Fusion Process of Oxygen, Hydrogen
and Water Molecules. New Energy Technologeis. 2003, Issue Nr 3 (12).
8. Кanarev Ph. M. Energy Balance of Fusion Processes of Molecules of Oxygen, Hydrogen and Water. http://kanarev.energy.innoplaza.net/
9. Ph.M. Kanarev, V.V. Podobedov. Device
for production of thermal energy and steam-and-gas mixture.
Patent No.
2157862.
10. Ph.M. Kanarev, E.D. Zykov, V.V. Podobedov.
Device for production of thermal energy, hydrogen and oxygen. Patent
No. 2157861.
11. Ph.M. Kanarev, V.V. Konarev, V.V. Podobedov,
A.B. Garmashov. Device for production
of thermal energy, hydrogen and oxygen. Patent No. 2175027.
12. Ph.M. Kanarev, V.V. Konarev, V.V. Podobedov. Device
for production of thermal energy, hydrogen and oxygen. Patent No. 2167958.
13. Ph.M. Kanarev, V.V. Konarev, V.V. Podobedov. Device
for production of thermal energy, hydrogen and oxygen. Patent No. 2167958.
14. Ph.M. Kanarev., Ya.A. Peysakhovich, V.V.
Podobedov. Device for production of thermal energy, hydrogen and oxygen. Patent
No. 2177512.
15. Ph.M. Kanarev, V.V. Podobedov, D.V. Korneev,
A.I. Tlishev, D.A. Bebko. Device for gas mixture production and transmutation
of the atomic nuclei of chemical elements.
Patent No. 2210630.
Webmaster: j_hartikka@hotmail.com
Generators of Global (Clean) Energy by Prof. Kanarev:
http://Kanarev.energygenerators.innoplaza.net
<< Kanarev΄s Page