ENERGY BALANCE OF FUSION PROCESSES OF THE OZONE MOLECULE
Ph.M. Kanarev, D.A.
Normov
The Kuban State Agrarian University. 350044 Krasnodar, 13, Kalinin Street, Russia
E-mail: kanphil@mail.kuban.ru
Abstract. It has
been disclosed that new physical chemistry of the micro world facilitates the description
of the ozone molecule fusion processes within the framework of the law of conservation of
energy.
Key words:
nucleus, atom, molecule, electron, photon, energy of bond.
Introduction
Ozone is a gaseous substance, which consists of three-atom molecules ![]() . In order to destroy the oxygen molecule, it is
necessary to spend 5.13 eV of
energy. During fusion of two ozone molecule, 2.99 eV of energy are released. As a result,
energy difference 5.13-2.99=2.15 eV takes place. The authors of the fundamental
monograph [1] devoted to ozone assert that energy of 2.15 eV is absorbed by the third
unknown particle
. In order to destroy the oxygen molecule, it is
necessary to spend 5.13 eV of
energy. During fusion of two ozone molecule, 2.99 eV of energy are released. As a result,
energy difference 5.13-2.99=2.15 eV takes place. The authors of the fundamental
monograph [1] devoted to ozone assert that energy of 2.15 eV is absorbed by the third
unknown particle ![]() , which takes part in
this process. The oxygen atom, the molecules of oxygen and ozone as well as any other
molecule being present in the ozone molecule fusion zone can play the role of this
particle. Such assumption is made for the purpose that the law of conservation of energy
will not be violated. The ozone molecule fusion reaction is written in such a way
, which takes part in
this process. The oxygen atom, the molecules of oxygen and ozone as well as any other
molecule being present in the ozone molecule fusion zone can play the role of this
particle. Such assumption is made for the purpose that the law of conservation of energy
will not be violated. The ozone molecule fusion reaction is written in such a way
![]() .
(1)
.
(1)
At any rate, it is a strange assumption. It is known that each portion of energy
has its owner in the processes of fusion and dissociation of the molecules. That’s
why it is necessary to find a true owner of energy of 2.15 eV [1], [2].
THEORETICAL PART
Prior to the analysis of energetics of the chemical bonds of the ozone molecule, it
is necessary to understand energetics of the chemical bonds of the atom ![]() and the oxygen molecule
and the oxygen molecule ![]() .
.
An oxygen atom is the eight element of the periodic table.
It is situated in its sixth group. The structure of its nucleus is given in Fig. 1, the
structure of oxygen atom is in Fig. 2 [2].
 |
Fig. 1. Diagram of oxygen atom nucleus: light
– the protons, dark and grey – the neutrons
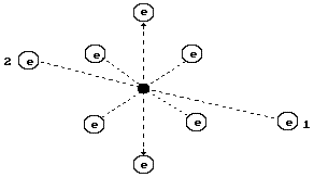
Fig. 2. Diagram of the oxygen atom
In Fig. 2, a diagram of the oxygen atom originating from
the structure of its nucleus is given (Fig. 1). It has eight electrons. The electrons
situated on the axis of symmetry are the most active ones (1, 2). Other six electrons
situated in the plane, which are perpendicular to an axis line (a line of symmetry), take
away electrons 1 and 2 from the nucleus at a large distance by their total electric field
forming the conditions for their large activity during the interaction with the electrons
of the neighbouring atoms [2].
The least ionization energy of the electron of the oxygen
atom is equal to ![]() =13.618 eV. Binding
energy of this electron with the atomic nucleus corresponding to the first energy level is
equal to
=13.618 eV. Binding
energy of this electron with the atomic nucleus corresponding to the first energy level is
equal to ![]() =13.752 eV. Let us call this electron the first one. The
calculation of energy indices of this electron, including its binding energies
=13.752 eV. Let us call this electron the first one. The
calculation of energy indices of this electron, including its binding energies ![]() with the atomic
nucleus according to the formulas (2) and (3), gives the following results (Table 1) [2].
with the atomic
nucleus according to the formulas (2) and (3), gives the following results (Table 1) [2].
![]() (2)
(2)
![]() (3)
(3)
Let us pay attention to the fact that there
is no energy of orbital movement of the electron in the atom in the mathematical model (2)
of the formation of the spectra of the atoms and the ions. It appears from this that the
electron has no orbital movement in the atom. Rotating in relation to its axis it can only
presess on the atomic nucleus (Fig. 2) [2].
Table 1. Spectrum
of the first electron of the oxygen atom
Values |
n |
2 |
3 |
4 |
5 |
6 |
|
eV |
10.18 |
12.09 |
12.76 |
13.07 |
13.24 |
|
eV |
10.16 |
12.09 |
12.76 |
13.07 |
13.24 |
|
eV |
3.44 |
1.53 |
0.86 |
0.55 |
0.38 |
The oxygen molecule structure is given in Fig. 3, a. It is formed by means of a
connection of unlike magnetic poles of axis electrons of two oxygen atoms [2]. As it is
clear, the oxygen molecule has fourteen free electrons, which are ready for bond. The
axial electrons 1’ and 2 are the most remote ones from the structure of the whole
molecule; they have the greatest activity, i.e. aptitude for bond with the electrons of
other atoms [2].
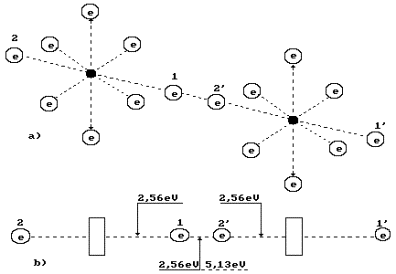
Fig. 3. Diagram of binding energy distribution between the
electrons in the oxygen molecule
It is known that the fusion process of the oxygen
molecules is accompanied with a release of 495 kJ/mole of energy, or in calculation for
one molecule
![]() (4)
(4)
What principle does the nature go by distributing energy
5.13 eV between the oxygen molecule electrons (Fig.
3, a)? Energy of 5.13 eV is a thermal binding energy between the electrons 1 and 2’
of two oxygen atoms (Fig. 3, a). When the oxygen molecule is formed, it is emitted in the
form of the photons by the electrons, which enter the bond. It appears from this that it
is equal to an amount of energies of two photons emitted by these electrons. Consequently,
each contacting electron emits a photon with energies of 5.13/2=2.565 eV (Fig. 3).
According to Table 1, in this case the valence electrons are situated between the second
energy level and the third one [2].
Two oxygen atoms are connected into a molecule in an
excitation state. The excitation state is the state of an atom when its valence electrons
are situated at such distances from the nuclei when the binding energy between them is
reduced to the thousandth fractions of an electron-volt. In such state, the atom can loose
an electron and become an ion. Otherwise, without loosing electrons it is connected with
an electron of the neighbouring atom by the valence electron, and a process of oxygen
molecule formation begins. It is an exothermic process when the axis valence electrons 1
and 2’ emit the photons, descend on lower energy levels and release 2.565x2=5.13 eV.
In order to destroy the oxygen molecule and to form the ozone molecule, spark
discharge or photon flux with energies, which are somewhat larger than binding energy of
2.565?2=5.13 eV between the oxygen atoms in its molecule, are used (Fig. 3, b). It is
known that ozone is formed during ultraviolet radiation with the wavelength of ![]() . Photon energy, which corresponds to this
wave length, is equal to
. Photon energy, which corresponds to this
wave length, is equal to
![]() (5)
(5)
As ozone is formed according to equation![]() , it is
necessary to destroy one oxygen molecule
, it is
necessary to destroy one oxygen molecule ![]() for fusion of two molecules of ozone
for fusion of two molecules of ozone ![]() . To this effect, it is necessary to excite 2 electrons
having spent 2.565x2=5.13 eV for this purpose (Fig. 4).
. To this effect, it is necessary to excite 2 electrons
having spent 2.565x2=5.13 eV for this purpose (Fig. 4).
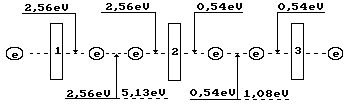
Fig, 4. Diagram of binding energy distribution in the
ozone molecule ![]()
It
is known that 144 kJ are released during dissociation one mole of ozone. As a result, we
have per molecule:
![]() (6)
(6)
Ozone formation process begins when even the smallest temperature reduction takes
place in ozone where the oxygen atoms are in excited state. Their valence electrons are
connected with the valence electrons of the oxygen atoms in its molecules and emit the
photons with such total energy that the remainder of energy absorbed earlier (5.13 eV)
will be equal to endothermic energy of 1.49x2=2.99 eV of formation of two ozone molecule.
Energy of the emitted photons will be equal to 5.13-2.99=2.15 eV. This energy is spent for
formation of the bonds in two ozone molecules, which have 4 valence electrons. Binding
energy corresponding to one electron is equal to 2.15/4=0.54 eV (Fig. 4). In this case,
valence electrons are almost on the fifth energy levels (Table 1).
As it is clear (Fig. 4), the ozone molecule is longer than the oxygen molecule
(Fig. 3); binding energy (0.54 eV) between the third one, which is connected by the oxygen
atom, is fivefold less than between the oxygen atoms (2.56 eV) in its molecule. As a
result, stability of the ozone molecule is less than stability of the oxygen molecule, and
it is destroyed easier forming the oxygen molecules and its atoms. For this effect,
availability of light photons, which energy is changed within the range of 0.016-3.27 eV,
is enough (Table 2).
Table 2. Electromagnetic emission scale ranges
Bands |
Wave-length, m |
Energy, eV |
1. Low- frequency
band |
|
|
2. Broadcast band |
|
|
3. Microwave band |
|
|
4. Relic band
(maximum) |
|
|
5. Infrared band |
|
|
6. Light band |
|
|
7. Ultraviolet band |
|
|
8. Roentgen band |
|
|
9. Gamma band |
|
|
After destruction of two ozone molecules, the valence electrons of the separated
oxygen atoms pass into exited state absorbing 0.54x4=2,15 eV of energy. When they reach
the highest energy levels, they are separated; after the free state stage, they form an
oxygen molecule emitting the photons with total energy equal to 5.13 eV. Difference
between emitted energy of 5.13 eV and energy of 2.15 eV absorbed by four electrons is
equal to the dissociation energy of two ozone
molecule of 2.99 eV, or 1.44 kJ/mole.
Conclusion
The disclosed models of the oxygen atom and molecule as well as empirical
mathematical law of formation of the spectra of the atoms and the ions give the
possibility to make a detailed analysis of energy balance of formation of the molecules of
oxygen and ozone, which corresponds to the experimental data completely.
References
1. V.V. Lunin, M.P. Popovich, S.N. Tkachenko. Physical chemistry of ozone. M. Publishing house of the Moscow University.1998. 475 pages.
2. Ph.M. Kanarev. The Foundation of Physchemistry of Micro World. Krasnodar. 2002. pag. 320.