Published 15.01.2003, updated 30.06.2003
ENERGY BALANCE OF FUSION PROCESSES OF MOLECULES OF OXYGEN, HYDROGEN AND WATER
Ph.M. Kanarev
The Kuban State Agrarian University,
Department of Theoretical Mechanics
13, Kalinin Street,
350044 Krasnodar, Russia
E-mail: kanphil@mail.kuban.ru
Abstract. A cause of additional energy appearance during
covalent bond formation in the fusion processes of the molecules of oxygen and
water has been disclosed, and the source of this energy has been established.
Key
words: nucleus, atom, molecule, electron, photon, bond
INTRODUCTION
In engineering
practice connected with ventilation system servicing, a phenomenon of excessive
thermal energy in circulating air has been found. Similar phenomenon has been
registered in water circulation systems with the devices for its active
cavitations. The results of our investigations explain not only a cause of
these phenomena, but they give an opportunity to perform quantitative
calculations of energy processes, which generate additional thermal energy [1],
[2], [3], [4], [5].
THEORETICAL PART
An oxygen atom is the eight
element of the periodic table. It is situated in its sixth group. The structure
of its nucleus is given in Fig. 1 [1], [2], [3].

Fig. 1. Diagram of
oxygen atom nucleus: light – the protons, dark and grey – the neutrons
In Fig. 2, a
diagram of the oxygen atom originating from the structure of its nucleus is
given (Fig. 1). It has eight electrons. The electrons situated on the axis of
symmetry are the most active ones (1, 2). Other six electrons situated in the
plane, which are perpendicular to an axis line (a line of symmetry), take away
electrons 1 and 2 from the nucleus at a large distance by their total electric
field forming the conditions for their large activity during the interaction
with the electrons of the neighbouring atoms [1], [2], [3].
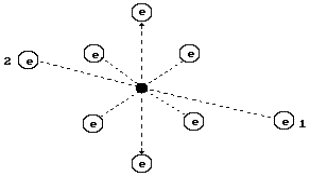
Fig. 2. Diagram of
the oxygen atom
The least
ionization energy of the electron of the oxygen atom is equal to ![]() =13.618 eV. Binding energy of this electron with the atomic
nucleus corresponding to the first energy level is equal to
=13.618 eV. Binding energy of this electron with the atomic
nucleus corresponding to the first energy level is equal to ![]() =13.752 eV. Let us call this electron the first one. The
calculation of energy indices of this electron, including its binding energies
=13.752 eV. Let us call this electron the first one. The
calculation of energy indices of this electron, including its binding energies ![]() with the atomic
nucleus according to the formulas (1) and (2), gives the following results
(Table 1) [1], [2], [3].
with the atomic
nucleus according to the formulas (1) and (2), gives the following results
(Table 1) [1], [2], [3].
![]() (1)
(1)
![]() (2)
(2)
Table 1. Spectrum
of the first electron of the oxygen atom
|
Values |
n |
2 |
3 |
4 |
5 |
6 |
|
|
eV |
10,18 |
12,09 |
12,76 |
13,07 |
13,24 |
|
|
eV |
10,16 |
12,09 |
12,76 |
13,07 |
13,24 |
|
|
eV |
3,44 |
1,53 |
0,86 |
0,55 |
0,38 |
The oxygen molecule structure
is given in Fig. 3, a. It is formed by means of a connection of unlike magnetic
poles of axis electrons of two oxygen atoms [1], [2], [3]. It is known that the
fusion process of the oxygen molecules is accompanied with a release of 495
kJ/mole of energy, or in calculation for one molecule
![]() (3)
(3)
What principle does
the nature go by distributing energy 5.13 eV between the oxygen molecule
electrons (Fig. 3, a)? Energy of 5.13
eV is a thermal binding energy between the electrons 1 and 2’ of two oxygen
atoms (Fig. 3, a). When the oxygen molecule is formed, it is emitted in the
form of the photons by the electrons, which enter the bond. It appears from
this that it is equal to an amount of energies of two photons emitted by these
electrons. Consequently, each contacting electron emits a photon with energies of 5.13/2=2.565 eV=![]() (Fig. 3). According to Table 1, in this case the valence
electrons are situated between the second energy level and the third one [1].
(Fig. 3). According to Table 1, in this case the valence
electrons are situated between the second energy level and the third one [1].
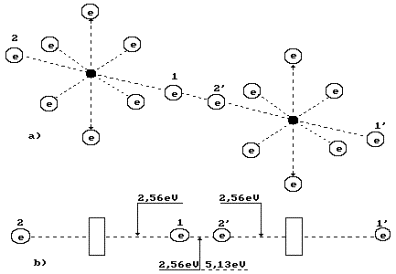
Fig. 3. Diagram of binding
energy distribution between the electrons in the oxygen molecule
Two oxygen atoms
are connected into a molecule in an excitation state. The excitation state is
the state of an atom when its valence electrons are situated at such distances
from the nuclei when the binding energy ![]() between them is
reduced to the thousandth fractions of an electron-volt. In such state, the
atom can loose an electron and become an ion. Otherwise, without loosing
electrons it is connected with an electron of the neighbouring atom by the
valence electron, and a process of oxygen molecule formation begins. It is an
exothermic process when the axis valence electrons 1 and 2’ emit the photons,
descend on lower energy levels and release 2.565x2=5.13 eV.
between them is
reduced to the thousandth fractions of an electron-volt. In such state, the
atom can loose an electron and become an ion. Otherwise, without loosing
electrons it is connected with an electron of the neighbouring atom by the
valence electron, and a process of oxygen molecule formation begins. It is an
exothermic process when the axis valence electrons 1 and 2’ emit the photons,
descend on lower energy levels and release 2.565x2=5.13 eV.
Let us pay
attention to the fact that energy 5.13 eV is released by two electrons, which
form a bond with energy of ![]() =2.56 eV. In modern chemistry, this bond is called a covalent
bond. In order to break this bond, it is necessary to use 2.56 eV of mechanical
energy. For thermal cleavage of this bond, double quantity of energy is
required, i.e. 5.13 eV. It is explained by the fact that the photon energy of
5.13 eV is absorbed by two electrons simultaneously. Only in this case, both
electrons will be transferred to the highest energy levels with minimal binding
energy
=2.56 eV. In modern chemistry, this bond is called a covalent
bond. In order to break this bond, it is necessary to use 2.56 eV of mechanical
energy. For thermal cleavage of this bond, double quantity of energy is
required, i.e. 5.13 eV. It is explained by the fact that the photon energy of
5.13 eV is absorbed by two electrons simultaneously. Only in this case, both
electrons will be transferred to the highest energy levels with minimal binding
energy ![]() when they are
disconnected, and each oxygen atom becomes a free one.
when they are
disconnected, and each oxygen atom becomes a free one.
Thus, energy
expenses for an oxygen molecule destruction depend on an impact method on the
bond. During thermal impact on the bond it is destroyed when energy is 5.13 eV.
During mechanical impact of the bond, it is necessary to spend 2.56 eV of
energy in order to destroy this bond. It appears from this that energetic of
fusion process of the oxygen molecule depends on its destruction method.
After the thermal
destruction of the oxygen molecule its formation process begins from emission
of the photons with energies of 2.56 eV by both valence electrons, and the
previous electrodynamics binding energy (![]() =2.56 eV) is restored between the electrons of both atoms.
=2.56 eV) is restored between the electrons of both atoms.
Thus, during the
thermal destruction of the oxygen molecule the same amount of thermal energy is
spent, which is released during its further formation. No additional energy
appears during thermal dissociation of the oxygen molecule and its further
fusion.
If the oxygen
molecule is destroyed by a mechanical method, it is necessary to spend 2.56 eV
of mechanical energy for this purpose. Valence electrons of the oxygen atoms
are in a free state by a lack of energy corresponding to such state as there is
no absorption process of 2.56 eV of energy by each of them. The electrons
cannot remain in such state, they should replenish immediately the energy,
which they have failed to receive during a mechanical break of the bond between
them. Where should they take it? There is only one source: environment, i.e.
physical vacuum filled with ether. They convert ether into energy of 2.56 eV
immediately. The next stage is a connection of two oxygen atoms, which valence
electrons have replenished the reserves of their energy at the expense of
ether. This process is accompanied by emission of the photons with energies of
2.56 eV by two electrons. Thus, energy of absorbed ether is converted into
thermal energy of the photons. If we spend 2.56 eV of mechanical energy for the
oxygen molecule destruction, we’ll get double quantity of energy (2.56x2=5.13)
eV during further fusion of this molecule. Additional energy is equal to 2.56
eV.
Many experimental
data show that in ventilation systems thermal energy of circulating air exceeds
electric energy spent for a fan drive. Now we know that this energy is
generated at mechanical failure of covalent bonds in the molecules of the
gases, which the air consists of.
Using the
above-mentioned method, we’ll analyse water molecule energetic, which sometimes
generates additional thermal energy. A water molecule consists of one oxygen
atom and two hydrogen atoms. Binding energies ![]() of the hydrogen atoms
with its nucleus are given in Table 2 [1], [2], [3].
of the hydrogen atoms
with its nucleus are given in Table 2 [1], [2], [3].
Table 2. Spectrum
of hydrogen atom
|
Values |
n |
2 |
3 |
4 |
5 |
6 |
|
|
eV |
10,20 |
12,09 |
12,75 |
13,05 |
13,22 |
|
|
eV |
10,198 |
12,087 |
12,748 |
13,054 |
13,220 |
|
|
eV |
3,40 |
1,51 |
0,85 |
0,54 |
0,38 |
It is known that a
connection of hydrogen with oxygen is accompanied by an explosion, but its cause
remains unknown. Let us try to find it.
Hydrogen molecule
fusion energy is equal to 436 kJ/mole, or 4.53 eV per molecule. As the molecule
consists of two atoms, the above-mentioned energy is distributed between them.
Thus, energy of one bond ![]() between the hydrogen
atoms is equal to 2.26 eV (Fig. 4). At thermal failure of this bond, double
quantity is required 2.26x2=4.53 eV [1].
between the hydrogen
atoms is equal to 2.26 eV (Fig. 4). At thermal failure of this bond, double
quantity is required 2.26x2=4.53 eV [1].
In order to form two water molecules, it is necessary
to break two hydrogen molecules and one oxygen molecule into atoms. If the
destruction processes of the above-mentioned molecules are carried out with a
thermal method, 4.53+4.53=9.06 eV are required for the destruction of two
hydrogen molecules, and 5.13 eV are required for the destruction of one oxygen
molecule. Totally, it is 14.19 eV. The difference between the energy spent for
mechanical and thermal destruction of a covalent bond of the molecules of
hydrogen and oxygen is almost double.
It is known that
during fusion of one mole of water 285.8 kJ or ![]() per molecule are
released. As a water molecule consists of one oxygen atom and two hydrogen
atoms, 2.96/2=1.48 eV falls per bond (Fig. 5). It appears from this that the
electrons of the atoms of hydrogen and oxygen in the water molecule are at the
usual temperature 1.48/2=0.74 eV =
per molecule are
released. As a water molecule consists of one oxygen atom and two hydrogen
atoms, 2.96/2=1.48 eV falls per bond (Fig. 5). It appears from this that the
electrons of the atoms of hydrogen and oxygen in the water molecule are at the
usual temperature 1.48/2=0.74 eV =![]() between the forth energy level and the fifth one (Table 2)
[1].
between the forth energy level and the fifth one (Table 2)
[1].
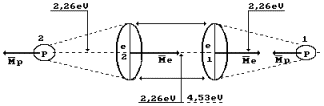
Fig. 4. Hydrogen
molecule
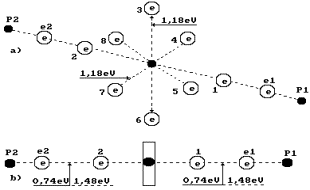
Fig. 5. Diagram of
water molecule:
1,2,3,4,5,6,7,8 are
the numbers of the electrons of the oxygen atom; P1, P2
are the nuclei of the hydrogen atoms (the protons); e1 and e2 are the numbers
of the electrons of the hydrogen atoms
Thus, when two hydrogen molecules 2![]() and one oxygen molecule
and one oxygen molecule ![]() are destroyed by the
thermal method, 14.19 eV are spent. As a result of fusion of two water
molecules (
are destroyed by the
thermal method, 14.19 eV are spent. As a result of fusion of two water
molecules (![]() ), 2.96x2=5.98 eV are released. It conflicts with the fact
that water molecule fusion process is an exothermic one with a release of 2.96
eV by one molecule. The given calculation shows that (14.19-5.98)/2=4.10 eV are
absorbed during fusion of one water molecule. What is the cause of this
contradiction?
), 2.96x2=5.98 eV are released. It conflicts with the fact
that water molecule fusion process is an exothermic one with a release of 2.96
eV by one molecule. The given calculation shows that (14.19-5.98)/2=4.10 eV are
absorbed during fusion of one water molecule. What is the cause of this
contradiction?
The oxygen atom in
the water molecule should reduce its volume when the transition from gaseous
state into liquid state takes place. It will happen when the rings electrons of
the oxygen atom descend on lower energy levels (nearer to the nucleus). They
will emit the photons, and we know their total energy. It is equal to energy
spent to destruction of two hydrogen molecules and one oxygen molecule, i.e.
14.19 eV. As two water molecules have 12 ring electrons, each of them will emit
14.19/12=1.18 eV=![]() (Fig. 5). It is more than axis electron binding energy (
(Fig. 5). It is more than axis electron binding energy (![]() =0.74 eV) with the nucleus, and it shows that the ring
electrons are situated nearer to the nucleus than the axis ones.
=0.74 eV) with the nucleus, and it shows that the ring
electrons are situated nearer to the nucleus than the axis ones.
In this case,
quantity of the energy produced due to fusion of two water molecules (14.19+5.98)
eV exceeds the energy spent for the destruction of two hydrogen molecules (9.06
eV) and one oxygen molecule (5.13 eV). The formed energy difference of 5.98 eV
is divided between two water molecules. It means that 5.98/2=2.99 eV or 285.8
kJ/mole fall per molecule. It corresponds to the existing experimental data
completely [1].
The above-mentioned
facts clarify a cause of the explosion, which takes place when hydrogen is
combined with oxygen. Simultaneous transition of six ring electrons of each
oxygen atom in the nascent water molecules to lower energy levels is
accompanied by simultaneous emission of the photons, which generate explosion
phenomenon.
Let us pay
attention to the fact that two binding energies ![]() between valence
electrons e2 and 2 and between 1 and e1 are shown in Fig. 5, b. Energy of one
electrodynamics bond is equal to
between valence
electrons e2 and 2 and between 1 and e1 are shown in Fig. 5, b. Energy of one
electrodynamics bond is equal to ![]() = 0.74 eV. If this bond is destroyed by the thermal method,
0.74x2=1.48 eV is required. This energy will be released during further fusion
of the water molecule from hydrogen atom
= 0.74 eV. If this bond is destroyed by the thermal method,
0.74x2=1.48 eV is required. This energy will be released during further fusion
of the water molecule from hydrogen atom ![]() and hydroxyl ion
and hydroxyl ion ![]() . In this case, no additional energy is generated.
. In this case, no additional energy is generated.
It appears from
this that the given bond is destroyed by the mechanical method spending 0.74 eV
per bond, each electron will have energy deficit equal to 0.74 eV after bond
failure. This energy will be absorbed from the environment immediately and will
be emitted during the repeated fusion of the water molecule from the hydrogen
atom ![]() and the hydroxyl ion
and the hydroxyl ion ![]() . At mechanical failure of one bond of water molecule, the
covalent chemical bond forms
. At mechanical failure of one bond of water molecule, the
covalent chemical bond forms ![]() = 0.74 eV of additional thermal energy, which is registered
in the water cavitation systems constantly (as we have already noted) [1], [2],
[3].
= 0.74 eV of additional thermal energy, which is registered
in the water cavitation systems constantly (as we have already noted) [1], [2],
[3].
It is known that
the water molecules unite and form clusters. If the bonds between the molecules
in the clusters are covalent ones, mechanical destruction of these bonds should
be accompanied by a release of additional thermal energy as well [1], [2], [3].
Experimental Part
Thus, chemical bonds between the atoms in the
molecules and the molecules in the clusters can be destroyed mechanically, by
electrodynamic and thermal impact on them. We have already shown that the
mechanical way of destruction of such bonds requires half energy as compared
with thermal energy. It appears from this that energy expenses for
electrodynamic destruction of these bonds should be less than thermal expenses
as well. Electrodynamic impact on the bond gives the opportunity to form the
resonance modes where energy expense for the destruction of these bonds is
reduced to greater degree. In order to check this hypothesis, a special
experiment was carried out connected with electrodynamic destruction of
chemical bonds of water molecules with a changing frequency of impact. The
check experiment in order to test this hypothesis was prepared and carried out
by (besides the author of this article) A.I. Tlishev, G.P. Perekotiy, D.A.
Bebko, D.V. Korneev. A diagram of the experimental installation is given in
Fig. 6. The results of this experiment
are given in Table 3.
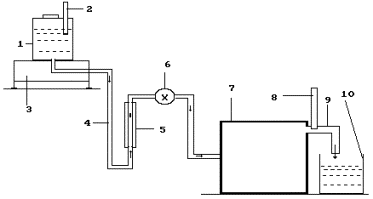
Fig. 6. Diagram of the experimental installation: 1 - reservoir
for solution; 2- thermometer; 3- electronic scales; 4 - solution feed duct; 5-
rotameter; 6- solution feed regulator; 7-a special thin plasma reactor is in
the process of patenting; 8 - thermometer; 9- heated solution discharge; 10-
inlet reservoir
PROTOCOL OF CONTROL TEST
|
Indices |
1 |
2 |
3 |
Mean |
|
1 – mass of the solution, which
has passed through the reactor m, kg. |
0.434 |
0.440 |
0.434 |
0.436 |
|
2 – temperature of solution at
the input of the reactor t1,
degrees |
24 |
24 |
24 |
24 |
|
3 – temperature of the solution
at the output of the reactor t2, degrees |
80 |
80 |
80 |
80 |
|
4 – temperature difference of the
solution Dt= t2 - t1,
degrees |
56 |
56 |
56 |
56 |
|
5 – durability of the
experiment Dt, s |
300 |
300 |
300 |
300 |
|
6 – reading of voltmeter V, V |
20.0 |
20.0 |
20.0 |
20.0 |
|
7 – reading of ammeter I, A |
1.40 |
1.40 |
1.40 |
1.40 |
|
8 – electric power consumption
according to indices of voltmeter and ammeters, E2=I×V×Dt, kJ |
8.40 |
8.40 |
8.40 |
8.40 |
|
9 – power spent for heating of
the solution, E3=4.19×m×Dt, kJ |
101.83 |
103.24 |
101.83 |
102.30 |
|
10 – reactor efficiency index K=
E3/ E2 |
12.12 |
12.30 |
12.12 |
12.18 |

Fig. 7. Photo of reactor heart
In the Russian
market, three firms (Yusmar, Termovikhr and Noteka) sell cavitation water
heating equipment with energy efficiency index of 150%. Soon, an air heating
device with the same efficiency will be produced. The processes of mechanical
destruction of covalent bonds of the air gas molecules, molecules and clusters
of water and their further fusion serve as a source of additional energy
generated by these devices [1], [2], [3].
CONCLUSION
Analysis of energy balance of the molecules with
covalent bonds shows the possibility of additional thermal energy formation
during mechanical and electrodynamics destruction of these bonds.
References
1. Ph.M. Kanarev. The
Foundation of Physchemistry of
Micro World. Krasnodar. 2002. 320 pages. (In Russian).
2. Kanarev Ph.M. The Foundation of
Physchemistry of Micro World.
The second edition. (In Russian). http://www.ikar.udm.ru/sb28-2.htm
3. Kanarev Ph.M.
The Foundation of Physchemistry of Micro World. The second edition. (In
English). http://book.physchemistry.innoplaza.net
4. Kanarev Ph. M.
Energy Balance of Fusion Processes of
Molecules of Oxygen, Hydrogen
and Water. (In English) http://Kanarev.innoplaza.net
5. Kanarev Ph. M. Energy Balance of Fusion
Processes of Molecules of Oxygen, Hydrogen and Water. (In Russian) http://www.n-t.org/tp/ts/eb.htm
http://Kanarev.energy.innoplaza.net
<< Kanarev´s Page